Mycoplasma testing is an essential step to detect any mycoplasma contamination during biopharmaceutical product manufacturing.
MycoFinder: sensitivity, simplicity, and speed
MycoFinder is a Real-Time PCR cell culture contamination detection kit that provides a sensitive, rapid, reliable, and cost-effective solution for the quality control of Advanced Therapy Medicinal Products (ATMPs) and biopharmaceuticals.
MycoFinder rapid mycoplasma cell culture contamination detection kit, has been validated in accordance with the EP 2.6.7, USP 63 and JP 17 and is available throughout EMEA.
MycoFinder uses real-time fluorescent PCR to ensure sensitive mycoplasma detection (below 10 CFU/mL). The kit is simple and quick to implement for product screening and batch release in laboratories where time is of the essence.
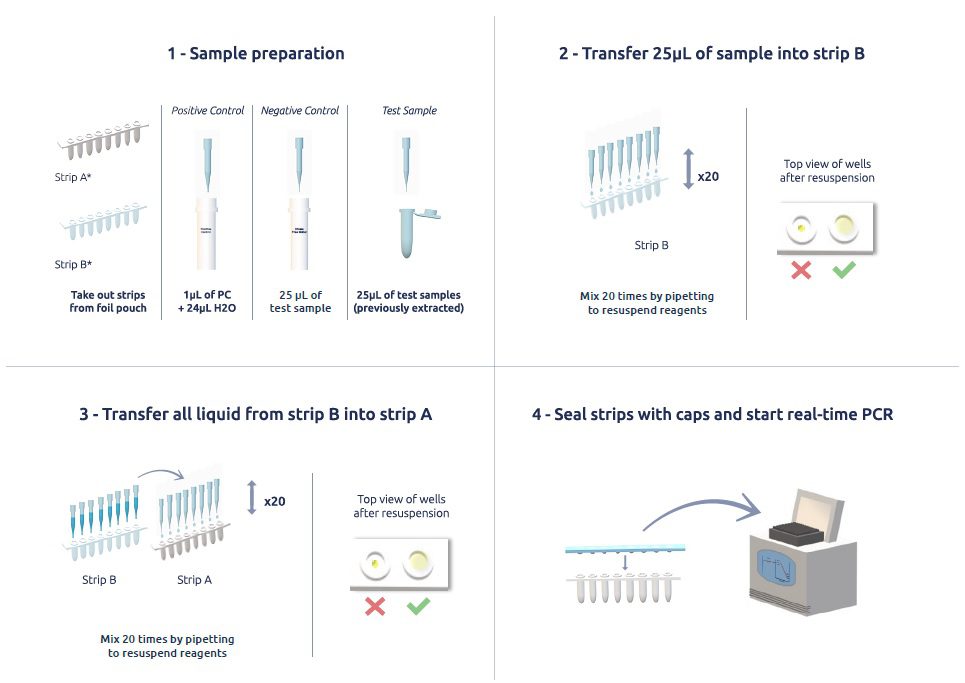
General protocol
MycoFinder is supplied with 2 different PCR tube formats (white and clear) which are compatible with most commercial PCR instrumentation.
Each PCR tube contains freeze-dried Master Mix thereby avoiding storage of different PCR reagents. MycoFinder is stored at 2° to 8 °C, and is ready to use, this makes our mycoplasma PCR test very simple and rapid to implement.
To prepare the PCR, the Master Mix is resuspended by pipetting 25µL of DNA sample directly into the tubes.
The sample prep workflow is completed within few minutes and provides PCR results in less than one hour, making MycoFinder one of the best mycoplasma detection kits available today.
Fully compliant with EP 2.6.7, USP 63 and JP 17 – our innovative mycoplasma test protocol reduces turnaround time and greatly eases sample preparation and handling.
PCR strip format – only need to use the number of PCR tubes required for your assay – reduces waste and cost.
Two different PCR tube formats (white and clear) compatible with most commercial PCR instruments.
Pre-mixed lyophilised reagents in each tube makes sample preparation quick and easy – Sample prep method completed within few minutes following a four-step protocol.